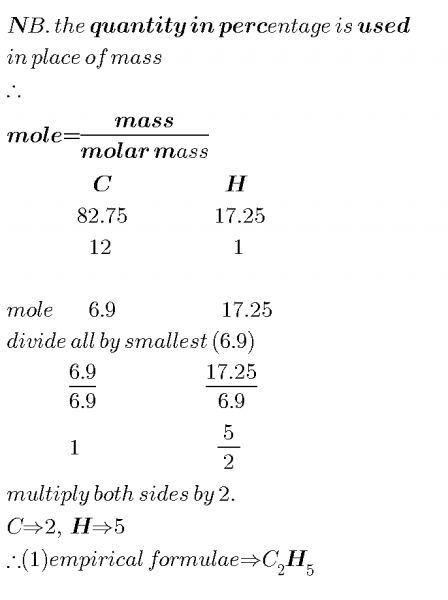
A gaseous hydrocarbon contain 82.75% carbon by mass.Given that the vapour density of the gas... - Myschool
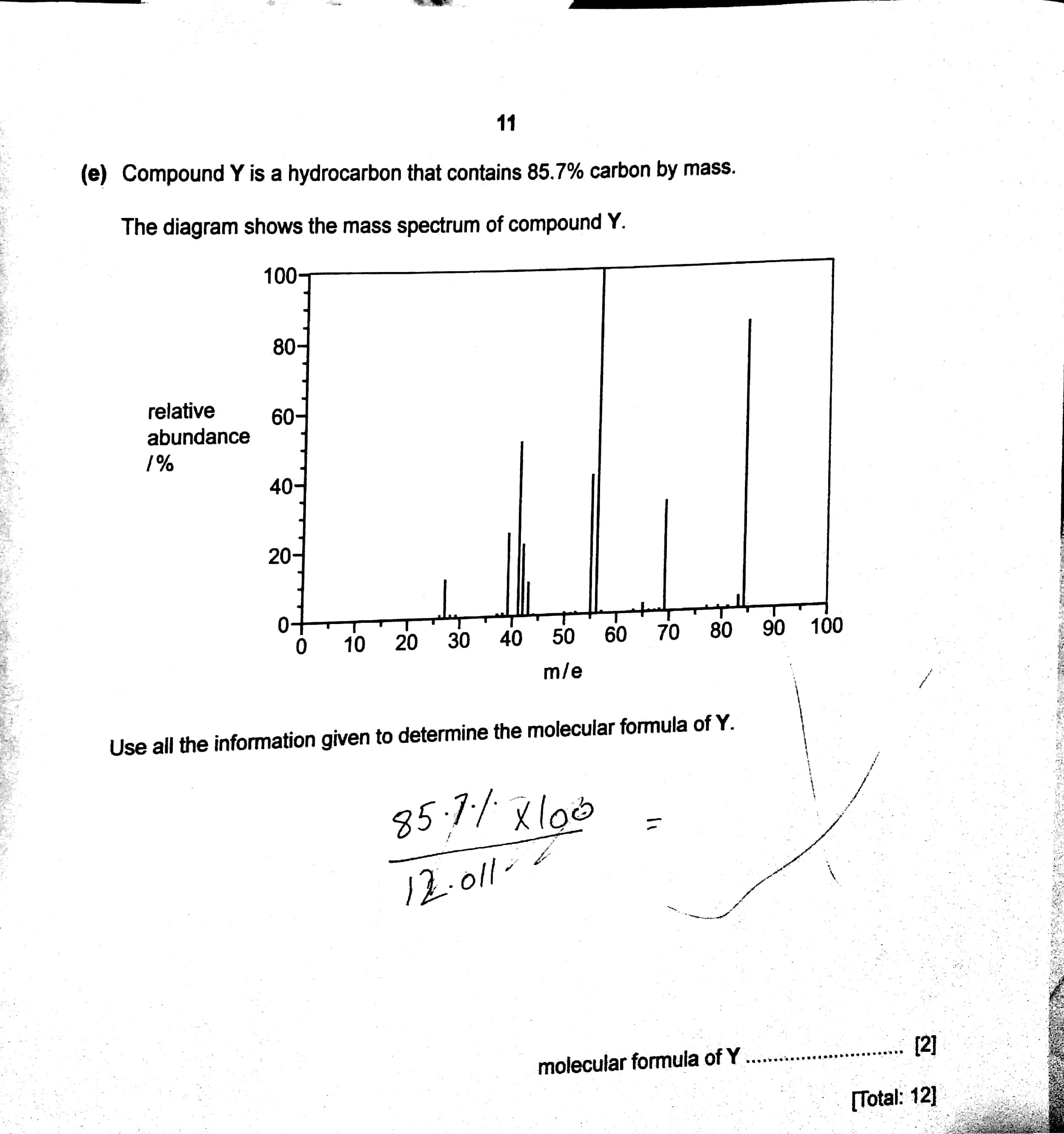
Compound Y is a hydrocarbon that contains 85.7% carbon by mass. The diagram shows mass spectrum of compound Y Determine molecular formula of Y? | Socratic
![A hydrocarbon contains 82.8% of carbon. Its molecular formula if its vapour density is 29 is : [H = 1, C = 12] A hydrocarbon contains 82.8% of carbon. Its molecular formula if its vapour density is 29 is : [H = 1, C = 12]](https://dwes9vv9u0550.cloudfront.net/images/8613707/64e4fea6-5f73-423f-8ccb-e406e23edbe3.jpg)
A hydrocarbon contains 82.8% of carbon. Its molecular formula if its vapour density is 29 is : [H = 1, C = 12]
A certain gases compound contains 82.8% of carbon and 17.2% hydrogen by mass. The vapour density of the compound is 29. What is the empirical formula? - Quora

A hydrocarbon contains 82.8% of carbon. Find its molecular formula if its vapour density is 29(H = 1, - Brainly.in

A hydrocarbon contains 82 8% carbon and has a relative molecular mass of 58 Write (i) Empirical formula, (ii) - Chemistry - - 4823654 | Meritnation.com
A hydrocarbon contains 82.8% of carbon. Find its molecular formula if its vapour density is 29 [H = 1, C = 12] - Sarthaks eConnect | Largest Online Education Community
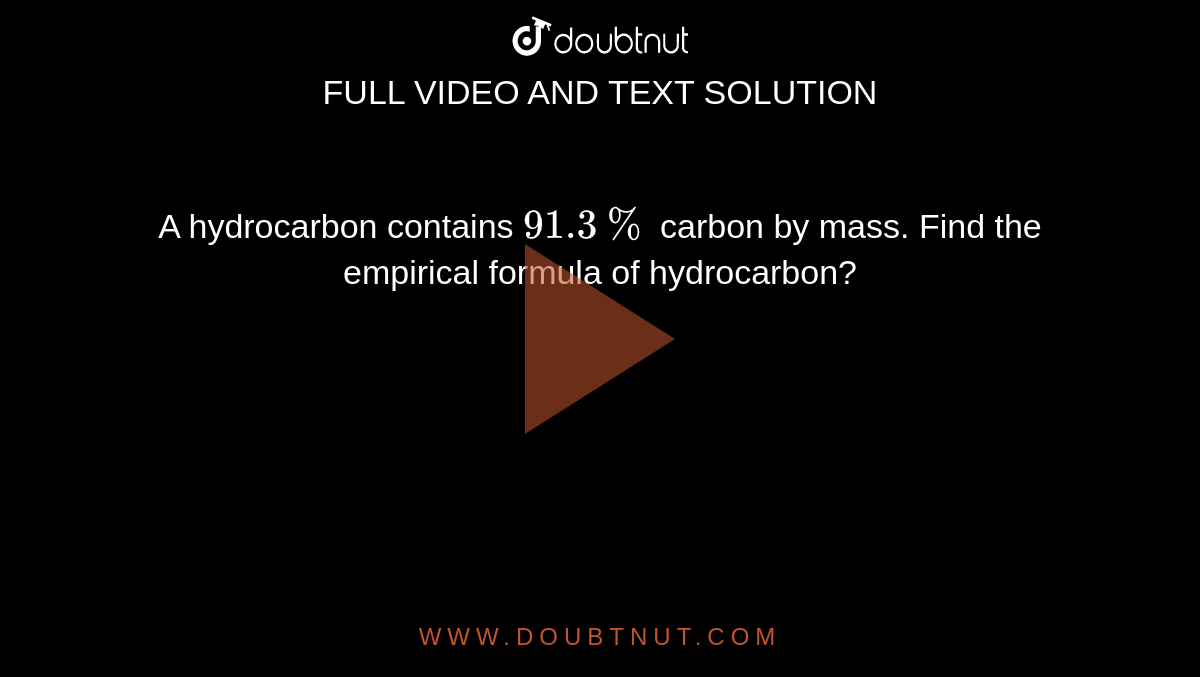
A hydrocarbon contains 82.8% of carbon and has a relative molecular mass of 58. Write its empirical formula.
A gaseous hydrocarbon contains 82.76% of carbon. Given that its vapour density is 29,find its molecular formula. [C = 12, H =1]. - Sarthaks eConnect | Largest Online Education Community
![A hydrocarbon contains 82.8% of carbon. Its molecular formula if its vapour density is 29 is : [H = 1, C = 12] A hydrocarbon contains 82.8% of carbon. Its molecular formula if its vapour density is 29 is : [H = 1, C = 12]](https://dwes9vv9u0550.cloudfront.net/images/10756423/2ad174d9-e115-4ed4-83ef-795347969ef5.jpg)
A hydrocarbon contains 82.8% of carbon. Its molecular formula if its vapour density is 29 is : [H = 1, C = 12]

Organic chemistry AS introduction first lesson empirical formula skeletal formula | Teaching Resources
![A hydrocarbon contains 82.8% of carbon. Its molecular formula if its vapour density is 29 is : [H = 1, C = 12] A hydrocarbon contains 82.8% of carbon. Its molecular formula if its vapour density is 29 is : [H = 1, C = 12]](https://dwes9vv9u0550.cloudfront.net/images/8760939/3c9ba6c8-1e90-4711-9fc8-6d0411305bed.jpg)











