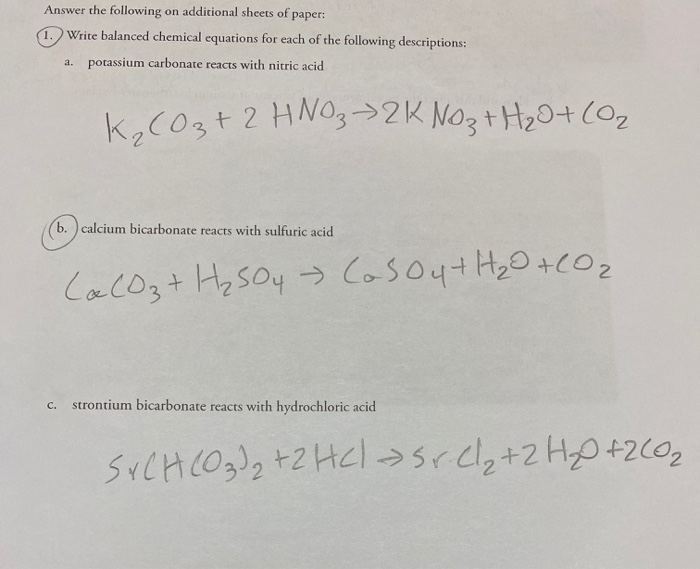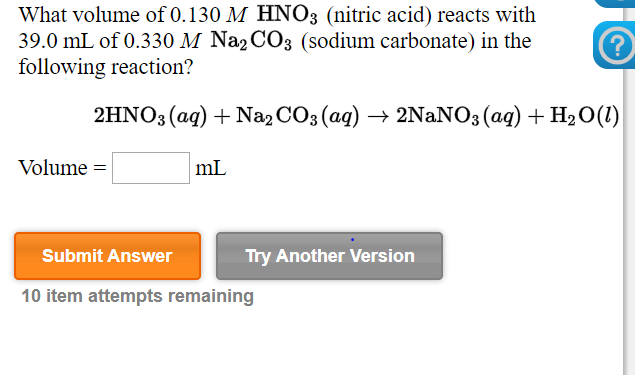Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa
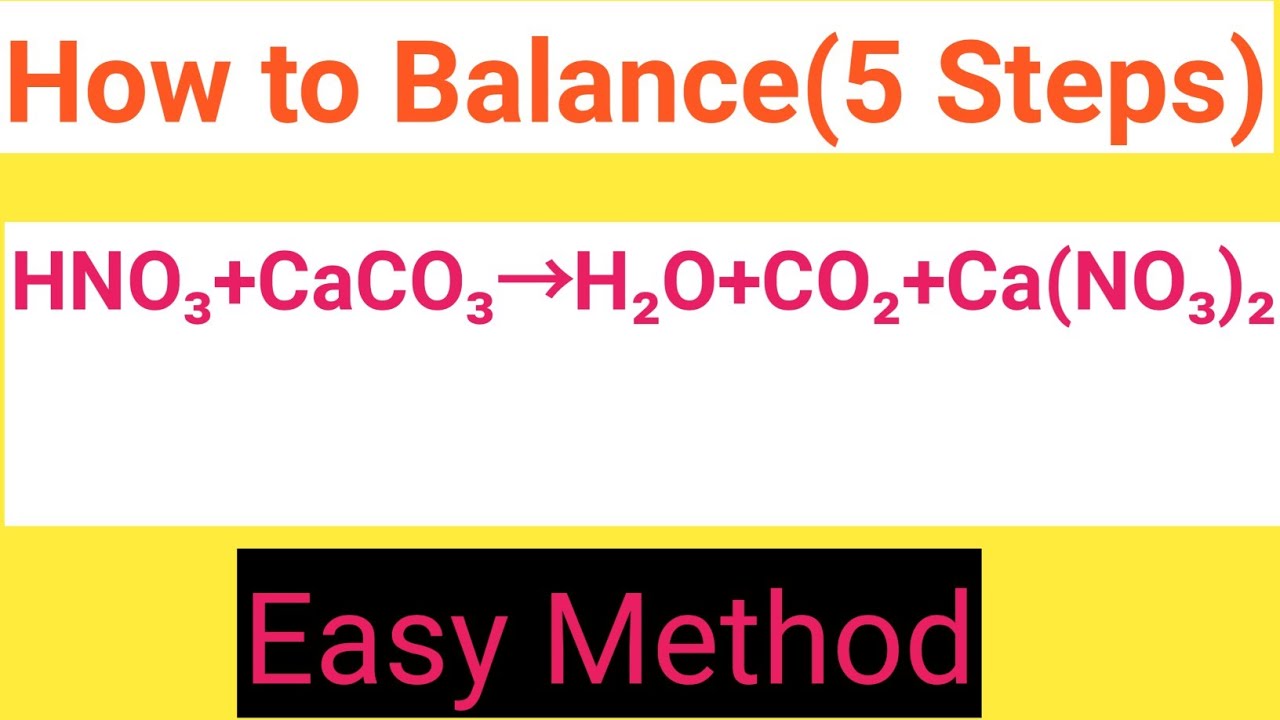
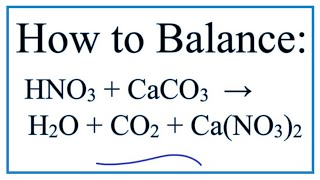
HNO3+CaCO3=H2O+CO2+Ca(NO3)2 Balanced Equation||Nitric acid+Calcium carbonate=Water+Carbon dioxide+ - YouTube
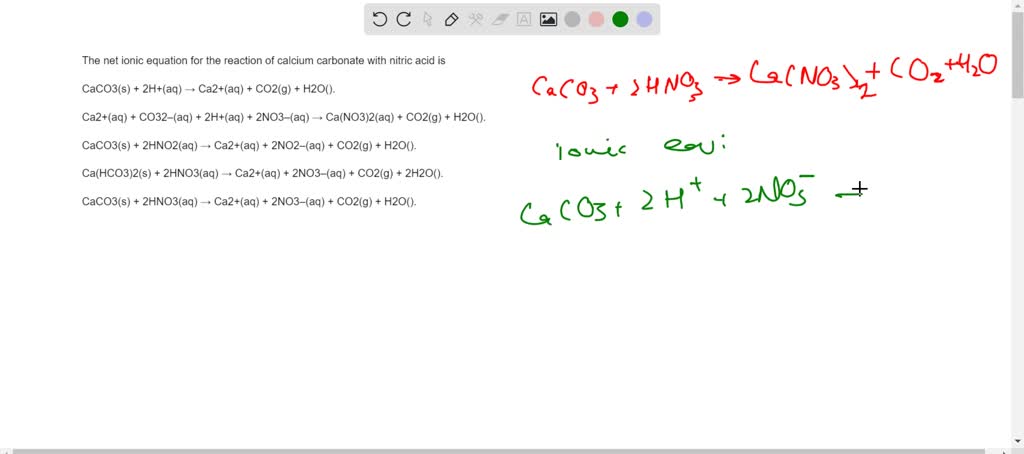
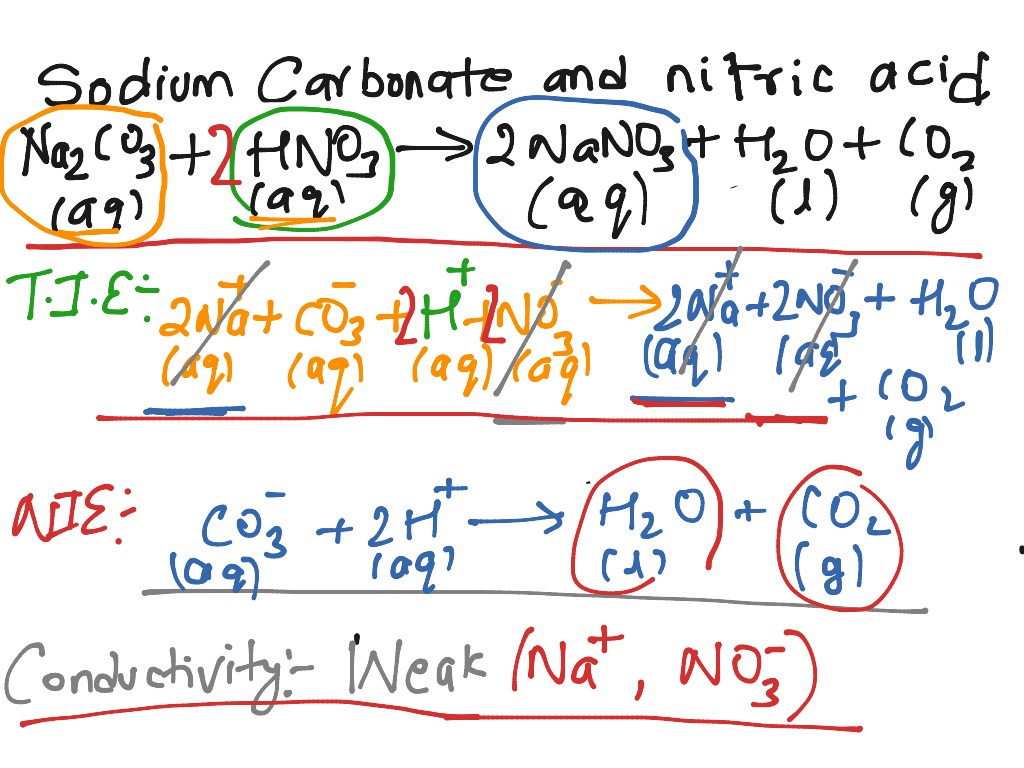
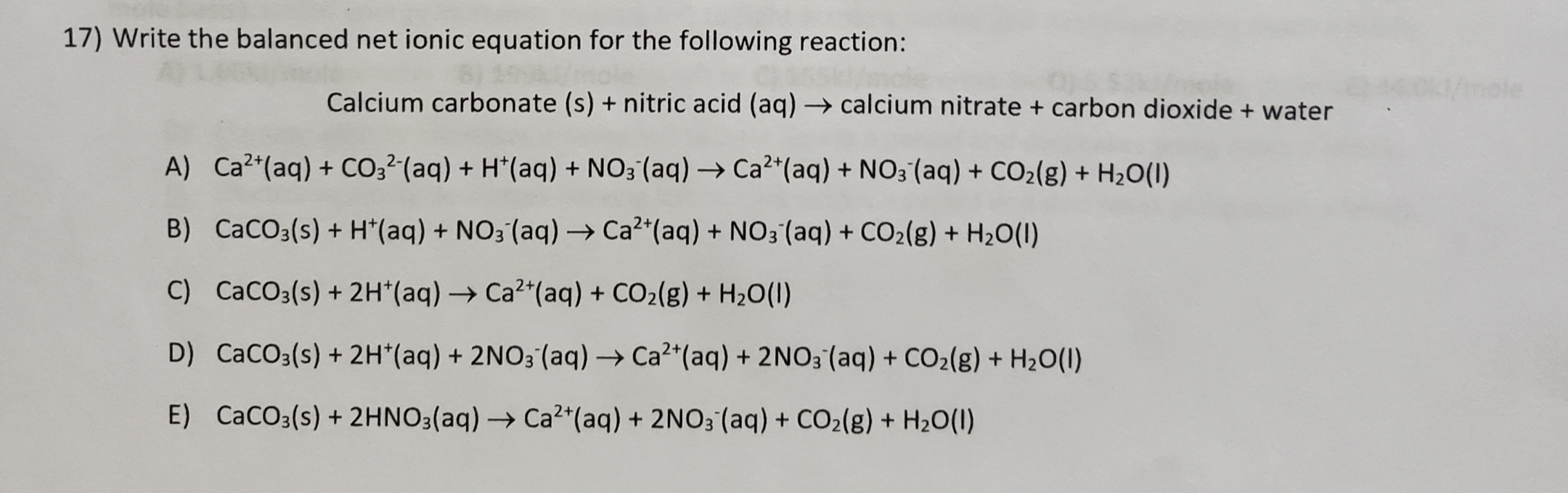
SOLVED: The net ionic equation for the reaction of calcium carbonate with nitric acid is CaCO3(s) + 2H+(aq) → Ca2+(aq) + CO2(g) + H2O(). Ca2+(aq) + CO32–(aq) + 2H+(aq) + 2NO3–(aq) →

Toward the Mechanistic Understanding of the Additives' Role on Ammonium Nitrate Decomposition: Calcium Carbonate and Calcium Sulfate as Case Studies | ACS Omega

OneClass: Write a net ionic equation for the reaction that occurs when calcium carbonate (s) and exce...
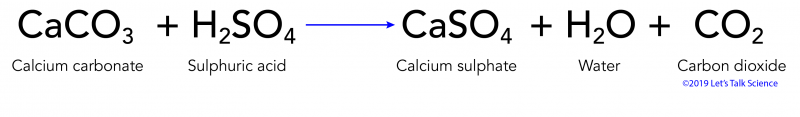
What is the balanced net-ionic equation for the gas producing reaction between hydrobromic acid and solid calcium carbonate? - Quora