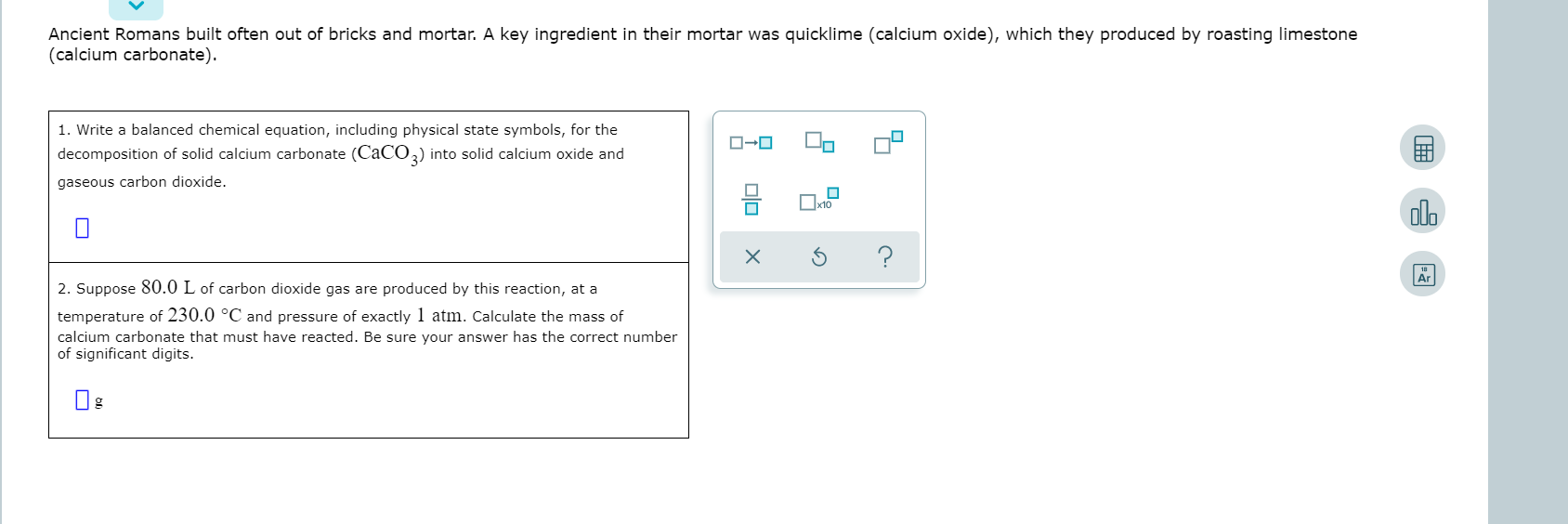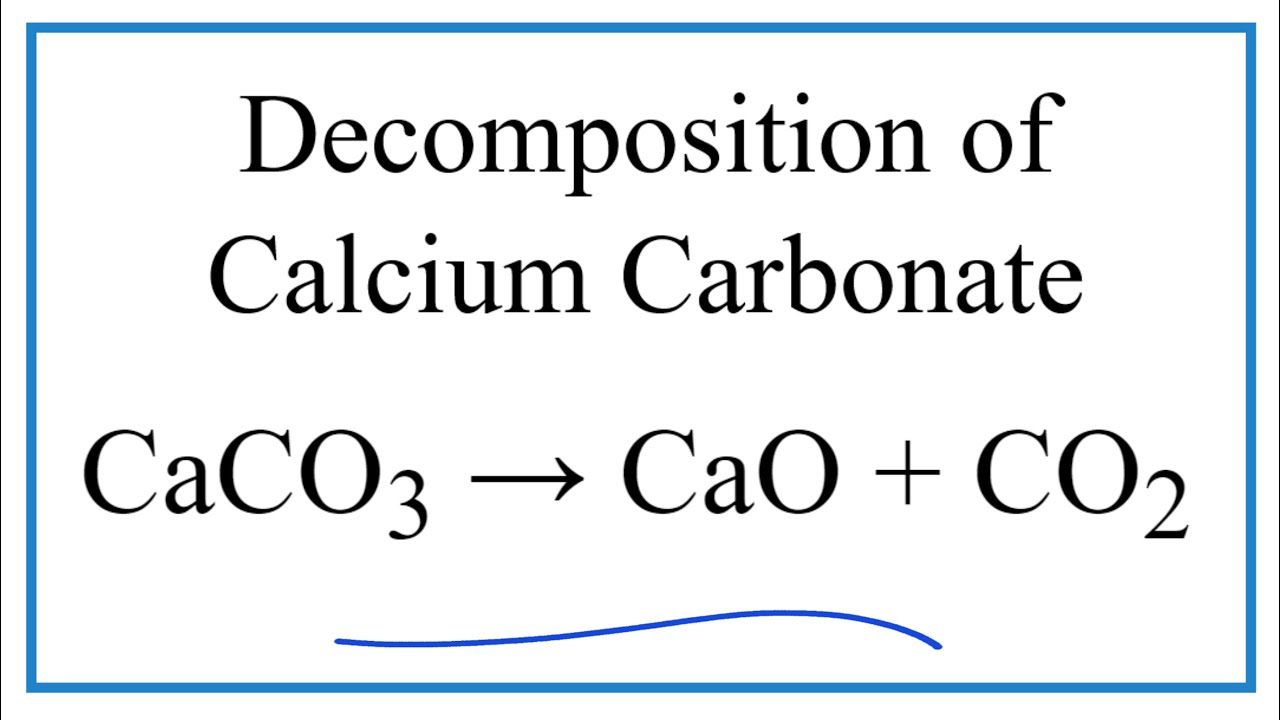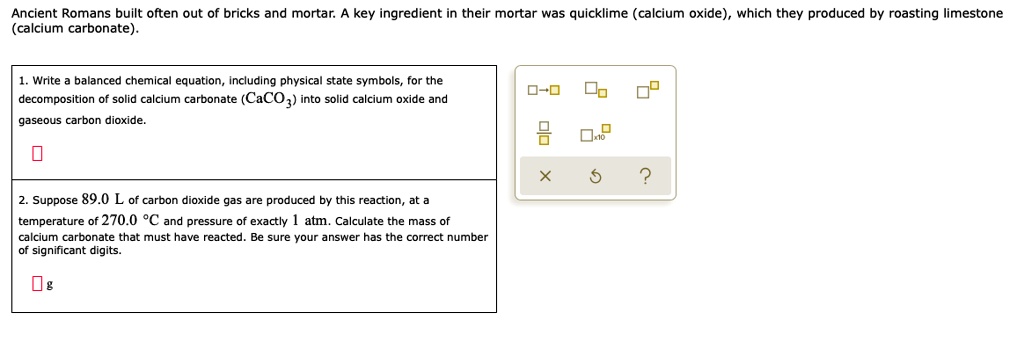
SOLVED: Ancient Romans built often out of bricks and mortar: key ingredient in their mortar was quicklime (calcium oxide) which they produced by roasting limestone (calcium carbonate). Write balanced chemica equation, Including

Question Video: Identifying the Chemical Equation- with State Symbols- That Corresponds to a Chemical Statement | Nagwa
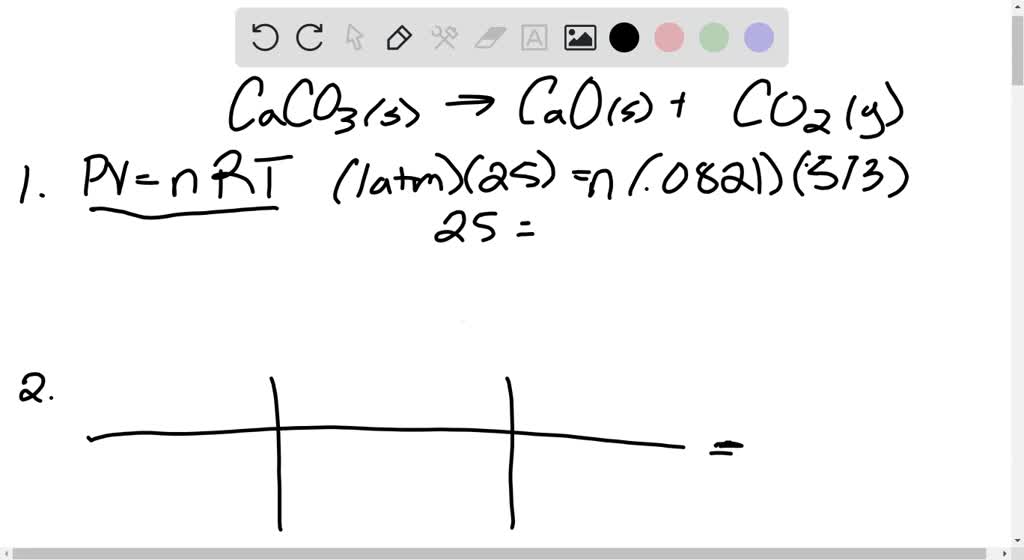
SOLVED: 1. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid calcium carbonate (CaCO3) into solid calcium oxide and gaseous carbon dioxide. 2. Suppose 25.0L of carbon

Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa
Limestone: Calcium Carbonate (CaCO3) - Uses, Preparation, Properties, Formula & Structure of Calcium Carbonate

Lakhmir Singh Chemistry Class 10 Solutions For Chapter 1 Chemical Reactions And Equations - Free PDF
What is the balanced net-ionic equation for the gas producing reaction between hydrobromic acid and solid calcium carbonate? - Quora

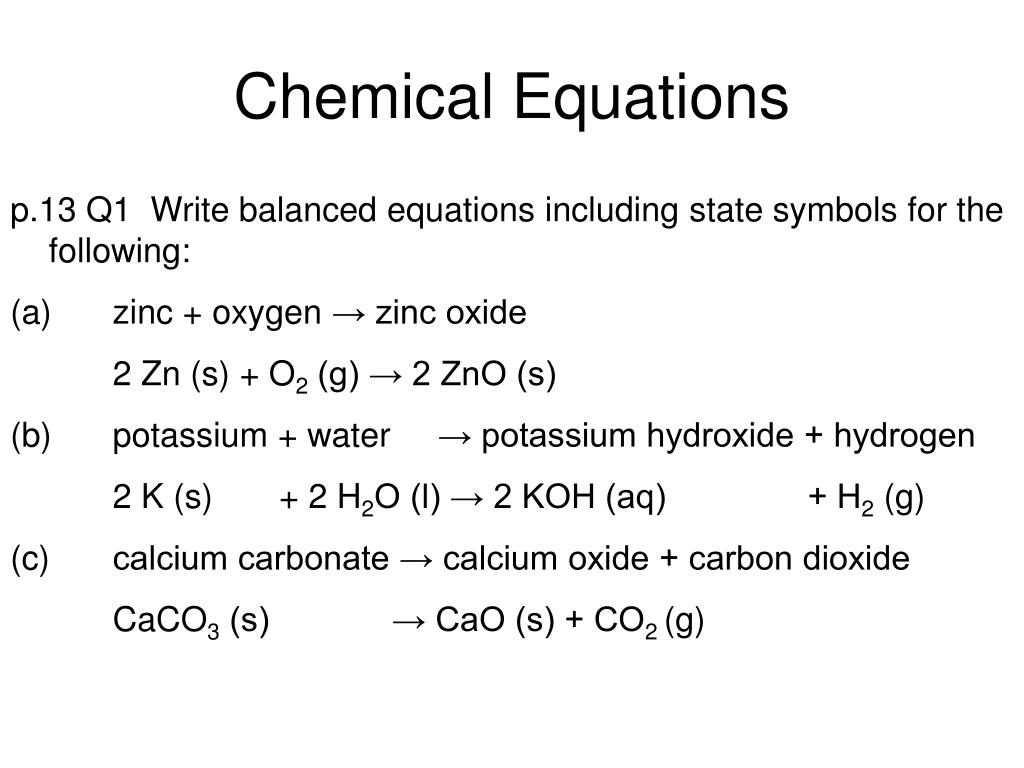
Cambridge International AS and A Level Chemistry: Coursebook with CD-ROM by Cambridge University Press Education - Issuu

Question Video: Calculating the Mass of Calcium Carbonate Required to Produce a Given Mass of Calcium Oxide | Nagwa